The Maillard reaction isn't unlike the economy.
It's the default explanation for a whole slew of questions, but it's shrouded in a veil of mystery, and most people don't seem to really understand very much about it (even if they pretend to).

Yet when you ask...
Why do the brown bits of roasted vegetables always taste the best? or
What's the point of getting a good sear on a steak? or
Why is it benefecial to smash potatoes and get a greater surface area for cooking them? or
Why does a fried egg taste different than a poached one?
...you get the same answer: It's the Maillard reaction, stupid!
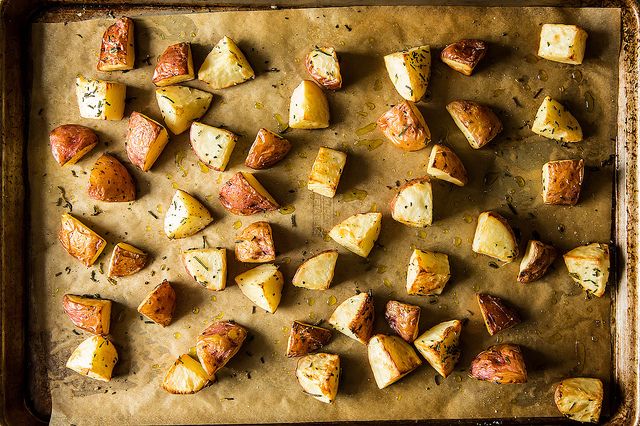
So what's the deal?
In layman's terms, the Maillard reaction, which was discovered in 1912 by Louis-Camille Maillard, is a series of chemical reactions between amino acids (the building blocks of proteins) and carbohydrates (the building blocks of sugars) that occurs when food—almost all types of food—is cooked.
The sear on your skirt steak? That's the Maillard reaction. The color of your toast? Maillard. The craggy, crisp bits on your fried egg? That's it, too.

Kenji J. López-Alt, food science expert at Serious Eats and author of The Food Lab, describes the process as a cascading waterfall. What starts out as a simple reaction between amino acids and carbohydrates becomes, as the folks at Modernist Cuisine explain, almost exponentially more complicated. The byproducts of early reactions continue to respond in increasingly complex ways, generating hundreds of new molecules, all of which contribute to the flavor and aroma of the food.
If all of this sounds really complicated, you're not alone: The reactions take place so quickly, with hundreds of relationships breaking and forming simultaneously, that, according to Kenji, much of what exactly goes on is still unknown. The Maillard reaction, at least at the microscopic level, is still somewhat of a mystery in food science, as perplexing as the processes of fermentation.

While it's not uncommon for the Maillard reaction to be conflated with "browning" in general, it's a misconception; there are many separate reactions that result in a similar change of color.
Take caramelization. The non-enzymatic browning reaction behind the brown top of crème brûlée and this richly flavored frosting occurs when sugars are oxidized. Unlike the Maillard reaction, it does not involve proteins. But to complicate matters, the two reactions frequently occur side by side. "Caramelized" onions, for example, have not exclusively undergone caramelization; while the sugars in the onions do oxidize, the proteins in the onions are also reacting with simple sugars in the Maillard reaction—both processes are happening at once, says Kenji.
And then there's charring and burning. At high enough temperatures, molecules are eliminated rather than generated. Yes, the food is becoming brown, but at this point, it's combusting as a source of energy, and you'll get harsher, simpler, and less tasty flavors.

How can you use the Maillard reaction to your advantage?
First off, cooking dryer food at higher temperatures results in more browning.
The Maillard reaction takes place more rapidly at a higher heat (that is, until you venture into burning territory). You'll want to aim for the 350° to 400° F range, where the rate of chemical reaction is faster and water evaporates more quickly. With the water out of the picture, less energy is expended on its evaporation and more goes into the Maillard reaction.
That's why you want to get your steak as dry as possible before adding it to a screaming-hot pan. And it's why Kenji recommends you leave cut vegetables uncovered in the refrigerator overnight; by drying them out, you're ensuring that they'll brown nicely when you roast them the next day (see the following photo for evidence!).
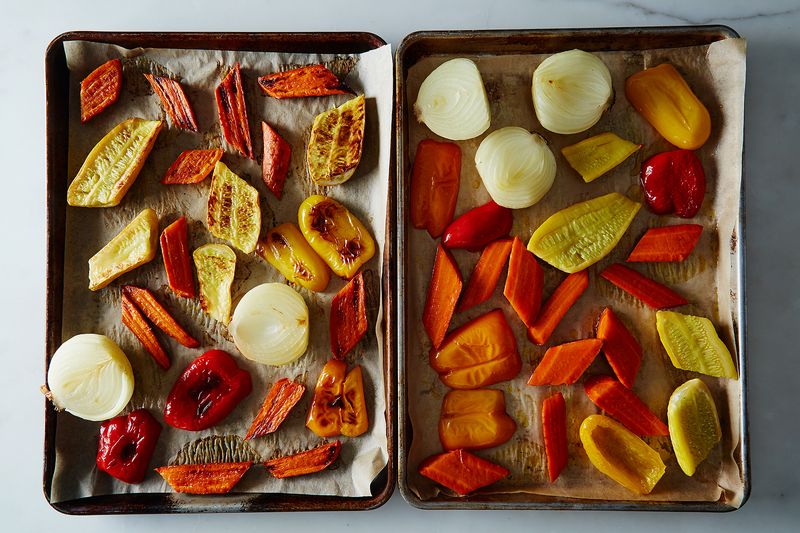
The vegetables on the left had sat, uncovered, in the refrigerator overnight before being roasted.
Second, the Maillard reaction occurs more quickly in alkaline environments (basic environments where the pH is above 7). Adding baking soda, a mild base, raises the pH of the food and will therefore speed up the Maillard reaction. Kenji's super-fast caramelized onions rely on the addition of 1/4 teaspoon baking soda to accelerate a process that's traditionally low-and-slow.
Baking soda can help you in other ways, too. Modernist Cuisine notes that Chinese cooks often marinate meat or seafood in mixtures containing egg white or baking soda immediately before stir-frying, and Kenji recommends rubbing chicken wings or porchetta with a mixture of salt and baking powder (like baking soda, it raises the pH) to achieve the often-elusive crispy skin.

So you may never know (or care to know) about all of the teeny-tiny reactions that make the brownest parts of your roasted cauliflower the most valuable. But you now know enough that the next time you scoop away all the crispy, burnished bits before your friends can get to them, you can look them in the eyes and say, with confidence, "It's the Maillard reaction, stupid!"
Photos by James Ransom and Bobbi Lin

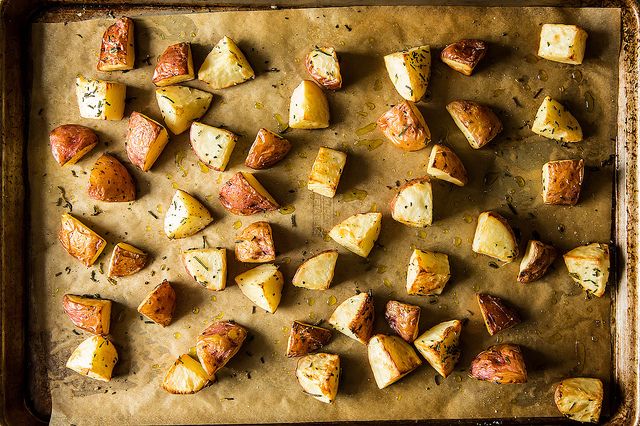



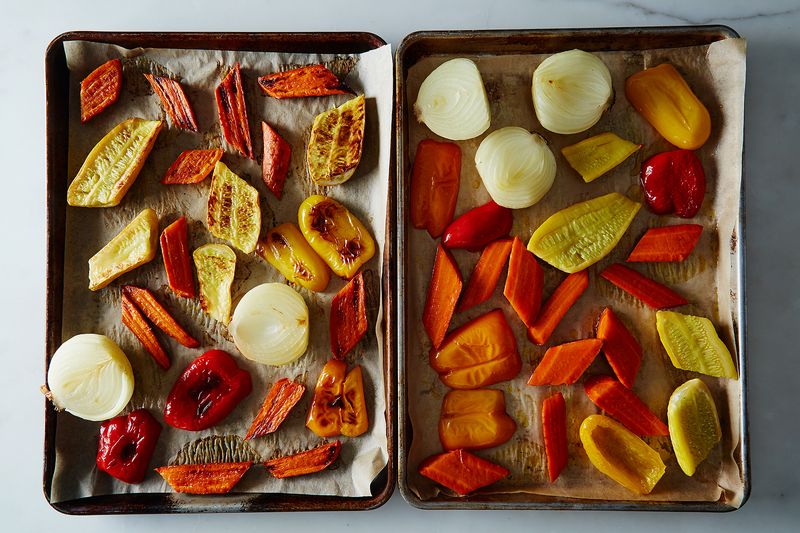


See what other Food52 readers are saying.